Our Technology
Photo-Defined Aperture Plate (PDAP)
PDAP is a patented, investigational approach to aerosolized drug delivery. Subject to regulatory approval, this medical aerosol technology will only be available as part of a drug/device combination product developed by or in partnership with Aerogen Pharma. This next-generation investigational vibrating mesh features the same metal alloy composition as the Vibronic® mesh found in the award-winning Aerogen® Solo hospital nebuliser, featuring a unique two-layer architecture with up to 24,000 precision-formed apertures. This enables independent specification of nebuliser droplet size and liquid output rate, creation of an aerosol plume of smaller droplet size and increased respirable fraction, and potential aerosolization of a broader range of challenging formulation characteristics.
PDAP’s potential advantages are on display in our AeroFact Development Program – where other technologies have failed, PDAP may help nebulise undiluted pulmonary surfactant, which possesses the problematic characteristics of high viscosity and low surface tension. The resulting ultra-fine, ultra-respirable aerosol, if approved by FDA, may help promote efficient lung deposition via non-invasive, trans-nasal administration in the smallest of preterm infants with tiny airways and challenging respiratory patterns. PDAP can potentially generate a full output within milliseconds, allowing the emission of aerosol at the beginning of even the fastest respiratory rates.
For more on our technology, and how to adopt it for your inhaled drug development program, please contact us directly.
The Problem
aiming to improve the efficiency of aerosol drug delivery
The efficiency and consistency of inhaled drug dosing in a hospital Intensive Care setting is often poorly defined, not least because it is highly variable, and may be profoundly influenced by many factors, including:
• ventilation modality and patient interface (e.g. endotracheal tube, nasal cannula, facemask)
• nebuliser type/technology (e.g. vibrating mesh vs. compressor “jet” vs. ultrasonic nebuliser)
• ventilator settings (e.g. gas pressure and flow)
• patient breathing parameters (e.g. tidal volume; breathing rate; inspiratory: expiratory ratio), pathology and cooperation
• nebuliser position in circuit (e.g. at ETT Wye adapter vs. proximally positioned in the inspiratory limb vs. prior to the humidifier chamber in actively humidified circuits)
• use of active, passive or no humidification
A study published in 2013 in the journal Respiratory Care showed the wide variation in aerosol delivery efficiency resulting from use of different nebulizer technologies and their positioning at different locations in the ventilator circuit.
This wide range of variation may be acceptable if the treatment paradigm is “dose to effect”, such as is typical of inexpensive short acting bronchodilators, but is not compatible with consistent, targeted delivery to the lung when precise delivery of effective dosing matters. Aerogen Pharma believes that high variability aerosol delivery methods increase risks and costs in the context of development of new drug/device combination products specifically aimed at efficacy in a critical care setting. It is vital to persuade regulators of dosing accuracy and reproducibility.
Our System
The Aerogen Pharma inhaled drug delivery system
Aerogen Pharma’s investigational product candidates are built on a combination of well-established drug molecules with a next-generation inhalation delivery system designed to achieve a high level of performance in a form that is easy to use with confidence. This system features a high-precision, single-patient-use custom vibrating mesh nebuliser, together with a respiration sensor and a re-usable controller, with the potential to facilitate breath-actuated drug delivery to patients of all ages and with different conditions. The delivery device is compatible with ventilatory support equipment used throughout the hospital, from intensive care units and operating rooms to emergency departments and wards. The system enables drug delivery to patients whether they are mechanically ventilated, on nasal cannula, or spontaneously breathing.
Designed to optimise lung delivery
Our own product development programs use this delivery system to repurpose generic drugs for the inhaled route of administration, generating a proprietary combination product with refreshed patent protection. We also offer the system to partners seeking a means of delivering novel biologics and small-molecule drug candidates to the lung. For such partner companies, the efficiency of this system may enable shortened treatment times and minimised consumption of expensive drug products such as proteins, nucleic acids and gene therapy vectors, while user-centric design features are aimed to promote acceptance and uptake by clinical personnel (see Partnerships).
Key features of the delivery system (if approved):
- • Ultra-respirable 2 micron aerosol and fast liquid output rate, enabled by the precision-engineered, breakthrough “PDAP” (Photo Defined Aperture Plate) mesh technology
• Designed for low variance, high accuracy drug delivery across a broad range of invasive and non-invasive ventilatory modes and parameters
• Light weight, orientation-independent aerosol source built directly into patient interface
• Aerosol synchronized with inspiration - boosts lung delivery to support efficiency, aiming for a lower nominal dose needed to reach the same efficacy with greater dose consistency
• Liquid drug feed to nebuliser on demand, eliminating the need for a gravity-dependent drug reservoir
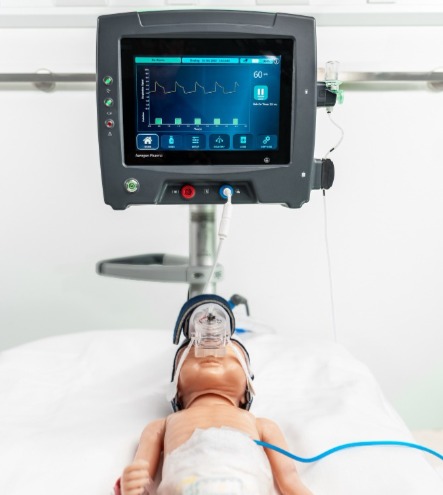
User friendly design
• Menu-driven, touchscreen interface that automates accurate patient dosing
• Custom interfaces for aerosol generation at the patient airway
• Equipped with alarms, dosage monitors, and other safety features
• Modular device design used with multiple Aerogen Pharma drug products
• Each drug is co-packaged with “plug-and-play” custom mesh nebuliser and interface suited to the targeted patient population / indication
• Provides clinicians with standardized and automated aerosol delivery.

Get in touch
To engage us on our technology, product pipeline,
clinical trials or partnerships, please provide
a few details below and we’ll have the right
Aerogen Pharma representative get in touch.

