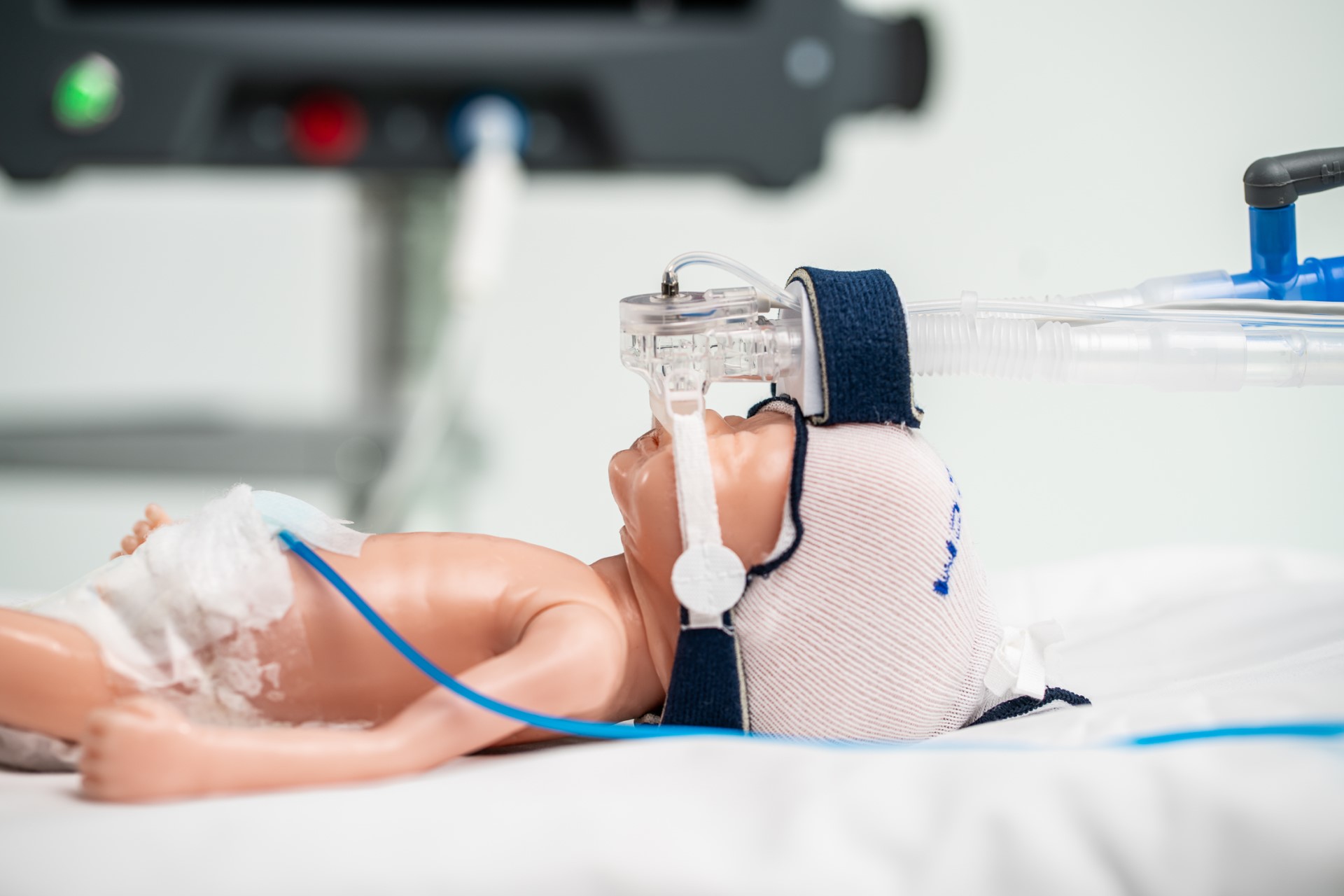
APC-0101 Overview
for treatment of respiratory distress syndrome (RDS) in premature infants
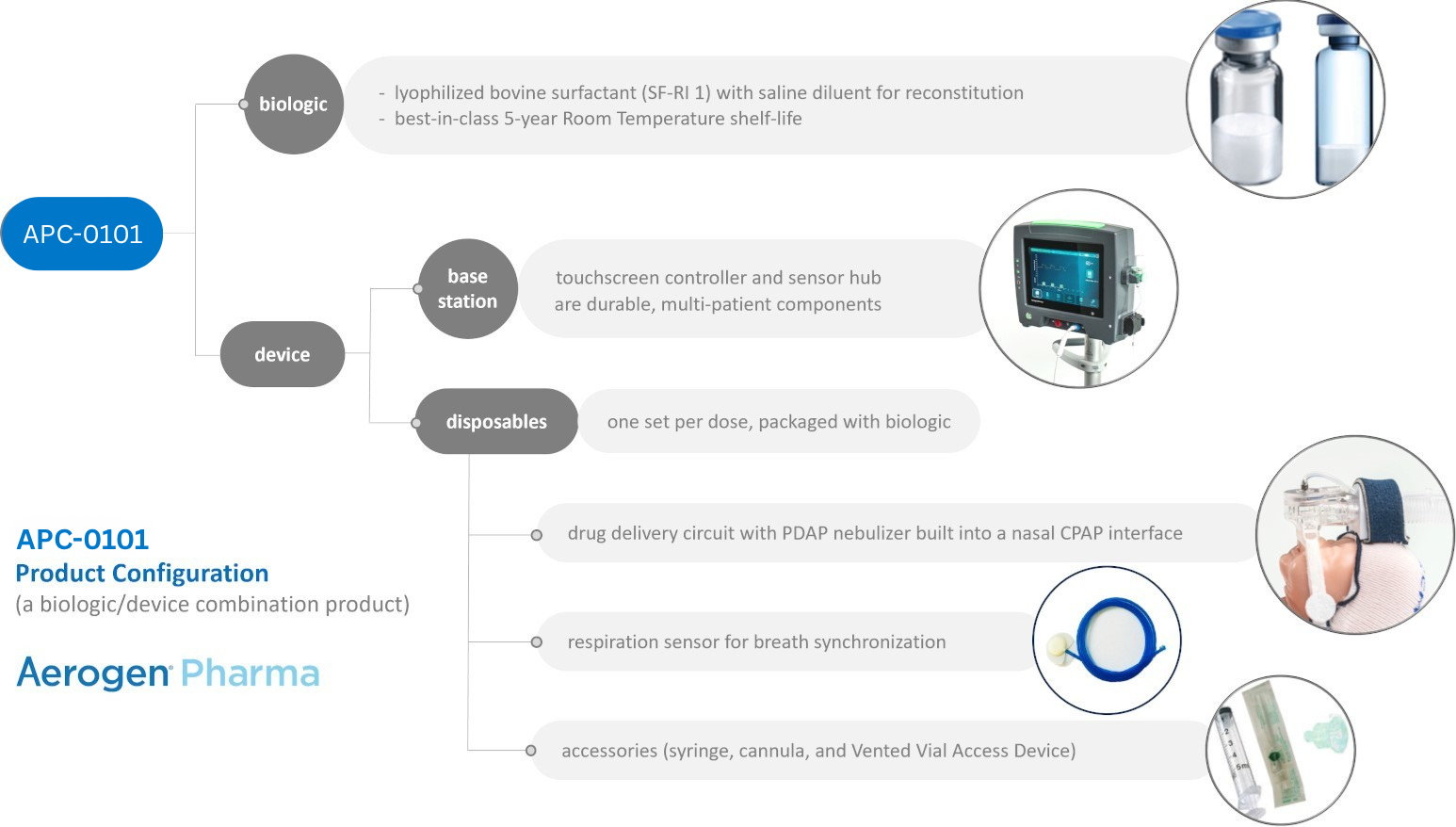
APC-0101 is
an investigational, biologic/device combination product, aiming to
deliver a major advance in the treatment of respiratory distress syndrome (RDS)
in premature infants. APC-0101 is designed to enable
trans-nasal pulmonary delivery of surfactant replacement therapy (SRT) to
infants on nasal continuous positive airway pressure (nCPAP) or non-invasive
ventilation (NIV).
APC-0101 is based on the combination of a bovine lung surfactant with a precise and highly efficient nasal delivery system.
Clinical Perspective
RDS is a complication of prematurity, caused primarily by the lack of pulmonary surfactant production by the immature lung. The risk of RDS increases as gestational age decreases. It occurs in approximately 1.4 million infants worldwide each year. In the US alone, an estimated 60,000 infants are born each year with RDS. Surfactant replacement therapy (SRT) has been a cornerstone
of neonatal intensive care since the 1980s and has had a remarkable effect on
the incidence of morbidity and mortality associated with preterm birth. SRT
relies on instilling a bolus of liquid surfactant into the trachea of the
infant, and can be accomplished by various techniques with varying degrees of
invasiveness (e.g. with intubation for ventilation, via INSURE, or via
LISA/MIST).
However, all these techniques require laryngoscopy to
visualize the vocal cords and placement of a tube through the vocal cords.
These techniques can be technically challenging, are frequently associated with
bradycardia and significant decrease in oxygenation, and cause discomfort for
the infant (unless performed with anesthesia). Due to the challenges associated with laryngoscopy and instillation of surfactant, multiple attempts have been made to treat RDS by delivering aerosolized surfactant to infants. However, despite more than 50 years of research, there are no approved or clinically effective approaches to delivery of aerosolized surfactant. Until now, major technical challenges have limited the delivery of an adequate dose of aerosolized surfactant to the alveoli: APC-0101 is an investigational biologic/device combination platform. The device component includes a vibrating mesh nebulizer which aerosolizes surfactant at the nasal interface and a controller which synchronizes aerosol generation with the infant’s inspiration. APC-0101 employs a combination of approaches to overcome the barriers of aerosolizing surfactant for the infant with RDS who is supported with NCPAP or non-invasive ventilation.:
APC-0101 Technical Features
Since pulmonary surfactant deficiency was first identified as the cause of RDS more than 60 years ago, major technical challenges have frustrated the development of a non-invasive surfactant delivery method:
- Aerosol droplets must be small enough to pass through the upper airways to deposit on the alveolar surface
- Aerosol must be generated as close as possible to the infant’s airway to minimize losses by deposition in the respiratory circuit or in bias flow gas
- Aerosol generation throughout the respiratory cycle is wasteful, since none of the drug aerosolized between inspirations is inhaled
Breakthrough technology achieves high-efficiency, cost-effective inhaled delivery for optimal lung dose efficacy
- Next-generation PDAP mesh technology turns undiluted surfactant into an ultra-fine, ultra-respirable aerosol plume with 1-3 μm droplets.
- Integration of PDAP nebulizer with a custom nasal interface design minimizes aerosol losses in the delivery circuit
- Synchronization of aerosol delivery with inspiration maximizes drug deposition and minimizes waste
Development Status
APC-0101 has received Fast Track, Rare Pediatric Disease and Orphan Drug designations from the FDA and EMA. A Phase 3 pivotal study is currently in design and is anticipated to begin in 2025.
Statutory Notice on Expanded Access Program under 21 CFR 312
Drug development programs in receipt of FDA Fast Track status are obligated to establish a policy concerning an Expanded Access Program. At this time, Aerogen Pharma is not offering access to APC-0101 outside of registered clinical trials.
Aerosolized Surfactant for Low- and Middle-Income Countries
Every year around the world, an estimated 1.4 million neonates develop respiratory distress syndrome (RDS), with two-thirds occurring in low- and middle-income countries (LMICs) where access to intensive care facilities is limited¹,². RDS is the leading cause of death among preterm infants in LMICs, with fatality rates reported to range from 57% to 89% ³. Although pulmonary surfactant was added to the WHO Essential Drug List in 2008, access to this gold standard treatment for RDS is limited in LMICs due to high drug costs, lack of personnel trained to administer surfactant, and shortage of the sophisticated medical equipment necessary to enable invasive mechanical ventilation and endotracheal instillation. One bright spot is that non-invasive CPAP ventilation is becoming more available in many LMICs as the primary mode of managing RDS ⁴. It is our belief that a surfactant delivered through CPAP ventilation has an important role to play in expanding the benefits of surfactant replacement therapy to a wider population.
Aerogen Pharma has received funding from the The Gates Foundation to develop Efficient, Safe, Feasible and Cost-Effective surfactant treatment for preterm infants in LMICs. Our shared goal is to see fewer families touched by the tragedy of infant mortality, and more preterm babies survive to enjoy full and productive lives.
-3muiyj.png)

Get in touch
To engage us on our technology, product pipeline,
clinical trials or partnerships, please provide
a few details below and we’ll have the right
Aerogen Pharma representative get in touch.